Introduction
Antithrombin is synthesised in the liver as a 464-amino acid protein but then undergoes cleavage at the N-terminus removing 32 amino acids to create a mature protein of 432 amino acids. Antithrombin contains 3 intra-chain disulphide bonds (-S--S-) between Cysteine [Cys] residues Cys8-Cys128, Cys21-Cys95 and Cys248-Cys430, a carbohydrate rich domain (CHO), an N-terminal heparin binding domain and a C-terminal serine protease binding domain.
α-Antithrombin is the dominant form of Antithrombin found in plasma and has an oligosaccharide occupying each of its four glycosylation sites at Asparagine residues 96, 135, 155, and 192. β-Antithrombin lacks glycosylation at Asn135.
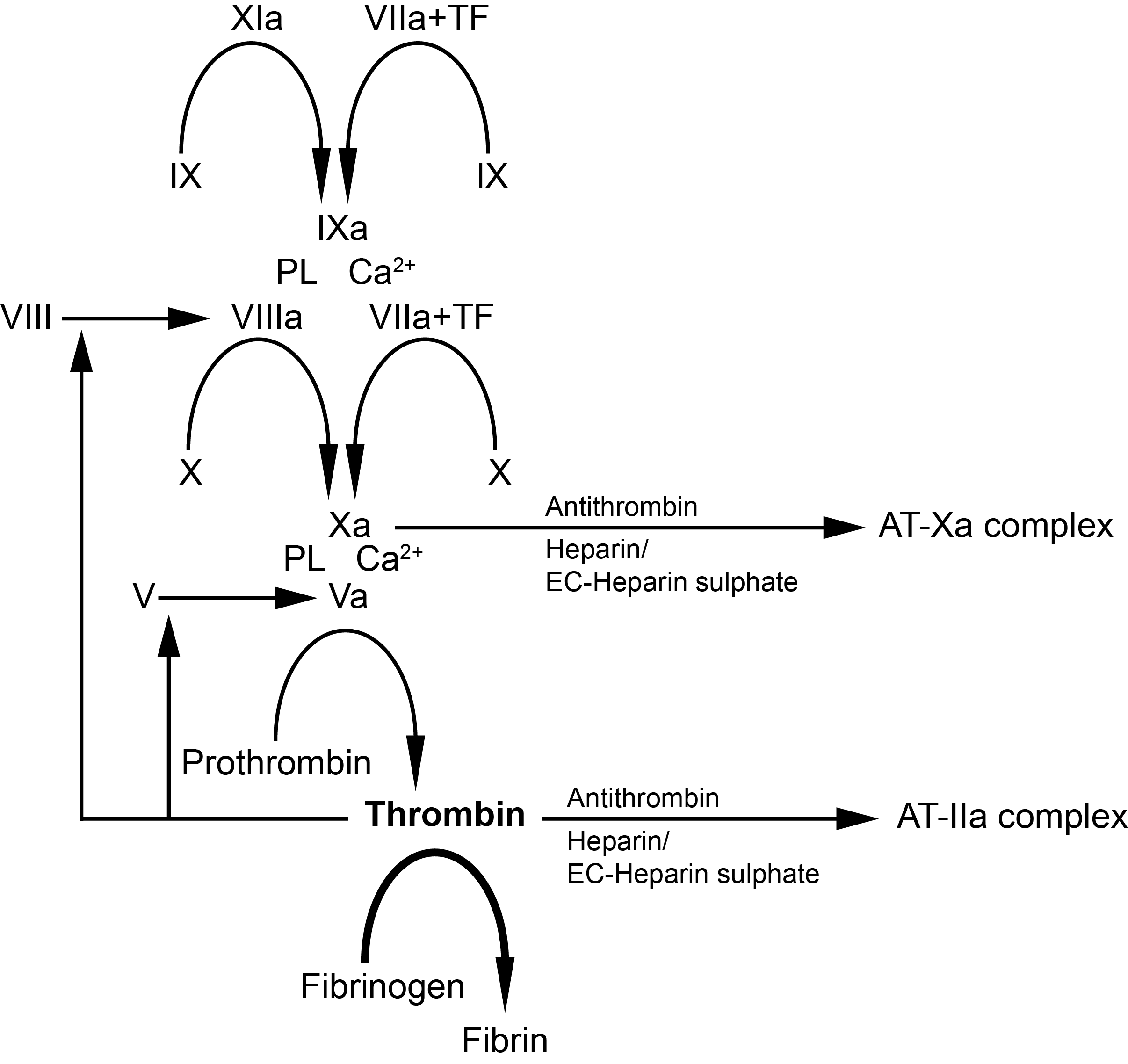
Heparin and Heparan Sulphate activate Antithrombin by binding to the D-helix of the protein through a common sequence-specific pentasaccharide and this induces a conformational change in the protein and its activation. However, for the efficient inhibition of Thrombin, a longer chain Heparin molecule that can form a bridge between Thrombin and Antithrombin, is required.
The inhibition of a serine protease e.g. Factor Xa, by Antithrombin after its activation, involves an interaction between the serine protease and a specific peptide bond [Arg393-Ser394] located within the Reactive Site Loop [RSL] of Antithrombin and which functions as a pseudo-substrate. Cleavage of this peptide bond by the serine protease traps the protease leading to its inactivation.
Antithrombin (AT) is a natural anticoagulant that plays a pivotal role in haemostasis by inhibiting the serine proteases Thrombin (IIa), Factor Xa (FXa) and to a lesser extent Factor XIa (FXIa), and Factor IXa (FIXa).
Inherited deficiencies of Antithrombin are associated with an increased risk of venous thromboembolic disease (VTE) and can be detected in 1-2% of patients with VTE as compared to 0.02-0.2% of the normal population. Inherited Antithrombin deficiency has a prevalence in the general population of between 0.2/1000 and 0.5/1000. Antithrombin deficiency is autosomally inherited and occurs in 1-5% of individuals with VTE.
Antithrombin is synthesised primarily in the liver and circulates in plasma as single chain protein of 432 amino acids with a molecular weight of 58,200 Daltons. The normal plasma level is 150 µg/mL and the plasma half-life is approximately 3 days.
The gene encoding AT [SERPINC1] is located on chromosome 1 and a wide variety of mutations have been identified in individuals with Antithrombin deficiency and venous thrombosis.
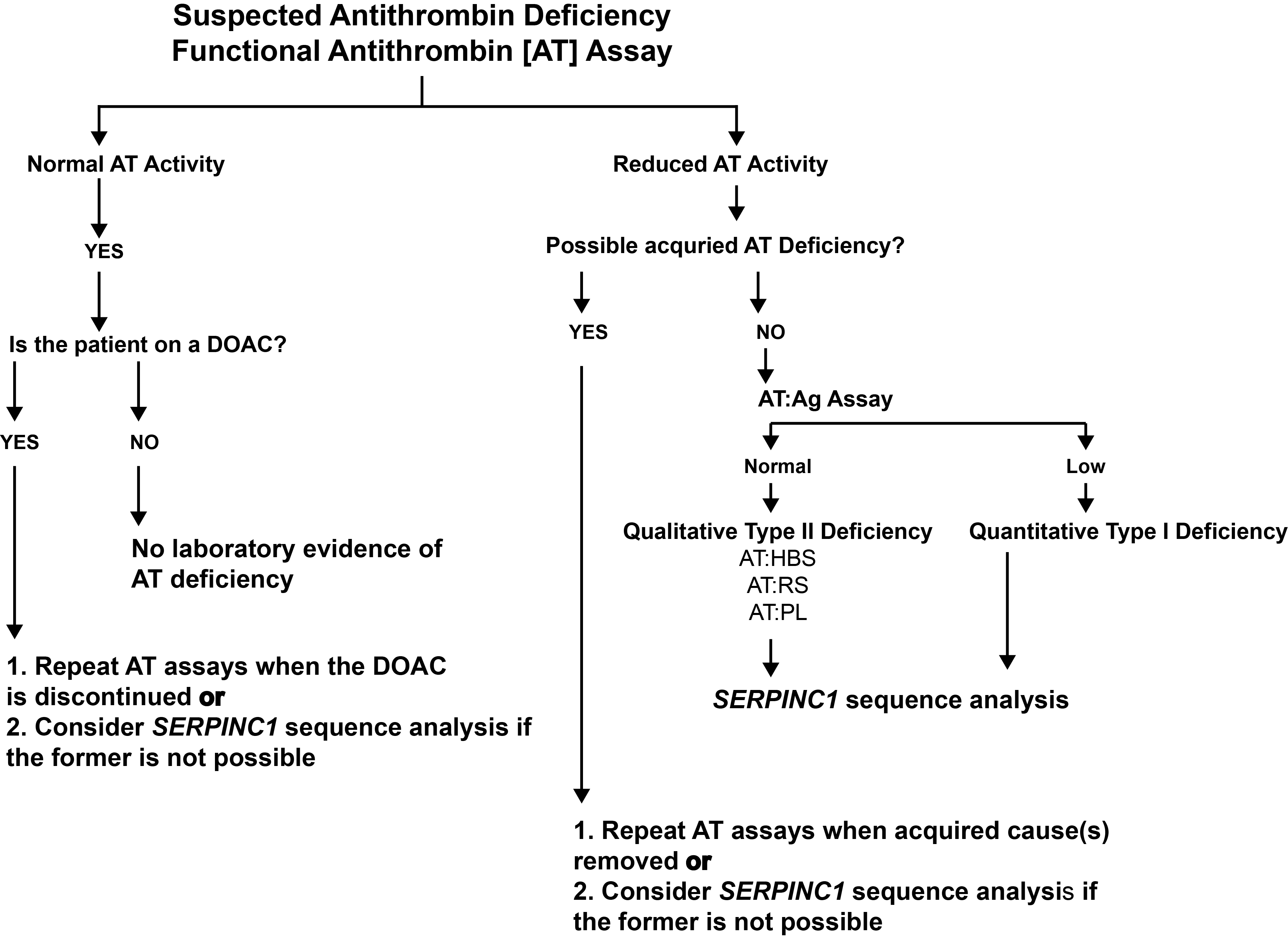
Plasma AT can be measured both by immunological (quantitative) or functional (qualitative) assays and it is on the basis of these assays that Antithrombin deficiency is classified.
Classification of Antithrombin Deficiency
| Type | Interpretation |
|---|---|
| Type I | Quantitative variants - Type I deficiency is associated with a parallel reduction in functional and immunological Antithrombin levels to approximately 50% of normal. |
| Type II |
Qualitative variants - Type 2 deficiency is associated with a greater reduction in the functional assay in comparison to the immunological assay |
Type II HBS:
Mutations affecting the heparin binding domain [site] of Antithrombin |
|
Type II RS:
Mutations that affect the reactive site of Antithrombin |
|
Type II PL:
Mutations that affect both the
heparin binding domain and the reactive site of the molecule i.e. Pleiotropic mutations. |
|
Principles & Method
Functional AT assays are based on the principle of FIIa or FXa inhibition by Antithrombin in the presence of heparin. The anti-FXa or anti-FIIa effect of AT can be measured by clotting or chromogenic assays although the latter is commonly used because it is more convenient.
1. Chromogenic Assays: Three chromogenic Antithrombin assays based on the inhibition of bovine FIIa, human FXa and human FIIa are available and all are based on a similar principle. Plasma is incubated with an excess of the relevant substrate [Thrombin or Factor Xa] in the presence of an excess of Heparin. The Heparin binds to and causes a conformational change in the structure of Antithrombin significantly increasing its inhibitory activity. A chromogenic substrate specific for the protease e.g. Thrombin or Factor Xa - is then added and any residual protease activity causes the cleavage of the substrate and a colour change. The absorbance measured at 405nm is inversely proportional to the AT activity concentration in the plasma sample.
| Substrate | Principles of the Test |
|---|---|
| Human IIa - Thrombin] | Plasma is diluted with saline and in the presence of Heparin incubated for 90 seconds with excess human FIIa forming an AT-Thrombin-Heparin complex. Excess human FIIa catalyses the release of p-nitroanaline (pNa) from a chromogenic substrate. The absorbance measured at 405nm is inversely proportional to the AT activity concentration in the sample. Heparin cofactor II can interfere with Human IIa-based assays and may lead to misleading results. |
| Bovine IIa - Thrombin | Plasma is diluted in saline and incubated for 30 seconds in a buffer containing bovine FIIa in excess and heparin sulphate. Residual bovine FIIa activity is measured using a FIIa sensitive chromogenic substrate. The rate of colour produced at 405nm is inversely proportional to the AT activity concentration. |
| Bovine Factor Xa | Plasma is diluted with saline and incubated for 90 seconds with an excess of bovine FXa in the presence of heparin. The residual FXa is measured by the rate of hydrolysis of the chromogenic substrate S-2765. The pNa released is measured at 405nm and is inversely proportional to the AT activity level in the sample. |
2. Immunological Assays:
| Test | Principles |
|---|---|
| a. ELISA Assays | AT antigen (AT:Ag) is commonly measured using a sandwich enzyme-linked immunoabsorbant assay (ELISA). Microtitre plates are coated with a Polyclonal anti-AT sera. Dilutions of the patients plasma, control plasma and a normal plasma pool standard are made, added to the plate and incubated at room temperature. Following a washing cycle the plate is tagged with an anti-AT horse radish peroxidase conjugated antibody. A substrate for the horse radish peroxidase is added and the absorbance of each well is measured at 490 nm. A standard curve can be constructed from the reference plasma and from which the AT activity of the unknown samples can be derived. |
| b. Laurell Electroimmunoassay | Historically Antithrombin [and indeed many proteins] were measured by electroimmunoassay [EIA] - so-called 'Laurell Rockets'. The principle is very simple: an antibody to the protein of interest [in this case Antithrombin] is mixed with molten agarose and poured into a U-shaped former clamped between two pieces of glass. After setting, the gel is adherent to one of the two sheets of glass. A row of 2-2.5mm wells approximately 1cm apart are punched into the gel. Into each well a 5µL aliquot of either the test plasma or a plasma standard is added. Each plate should include a series of doubling dilutions of the plasma reference standard from the which the standard curve is constructed. The plate is positioned in an electrophoresis tank so that the direction of current flow is at right angles to the row of wells. Wicks are placed at each end of the gel, electrophoresis is carried out with constant current for 14-18 hours. At the end of the period, the plate is washed, blotted dry and the antibody-antigen complexes detected by staining in Coomassie Blue - see below. |
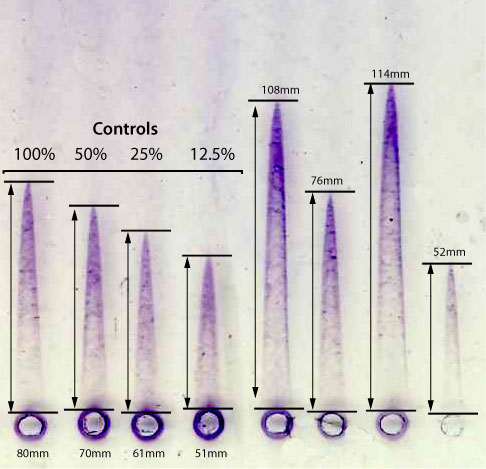
A standard curve is constructed by plotting the peak heights of a series of dilutions of a reference plasma. The rocket height (mm) is plotted on the linear X-axis and the concentration on the logarithmic Y axis [see below.] From this graph the concentration of the unknowns can be derived e.g. a peak height of 108mm corresponds to an AT:Ag concentration of 720%. The other unknown plasma samples can be calculated using the same method.

Interpretation
There is a clear difference in the ability of the various AT assays to detect all variants of Antithrombin. The bovine FIIa assay appears to be the most sensitive assay and with a short incubation time in the assay appears to be able to detect all Antithrombin variants including heparin binding variants. In contrast, the human FIIa and bovine FXa assays may fail to detect significant numbers of clinically important variants. The difference in the sensitivity of the three assays may reflect the underlying molecular defect, the presence of other serine protease inhibitors, or variations in the assay incubation time.
Acquired Antithrombin deficiency has been reported in a number of settings:
- Liver dysfunction
- Consumption as in Sepsis/DIC, Pre-eclampsia, AML
- In association with L-Asparaginase
- Proteinuria
- Heparin therapy may lead to a reduction in Antithrombin levels
- Active Crohn's and Ulcerative Colitis
- Poor nutrition
- Dilutional - Haemodialysis, Cardio-pulmonary bypass, Plasmaphoresis
Reference Ranges
Neonates have reduced levels of Antithrombin at birth (30-50% of adult levels: range 39-87 U/dL) but they rise to approximately 60% of that of adult levels 1 month after birth and reach adult values by the age of 6 months.
The adult reference range for Antithrombin in men is 74-126 IU/dL and in women very similar at 71-130 IU/dL.
What Test Next
In individuals with a low Antithrombin assay, the test should be repeated to confirm the low result and after any illness than might lead to a low result. If the low assay is confirmed, it important to establish whether this is due to a Type I or Type II mutation and whether the mutation affects the heparin binding site, the reactive site or has pleiotropic effects. This is generally achieved by sequencing the Antithrombin [SERPINC1] gene. Informed genetic counselling can be provided on the basis of the mutation [or similar mutations].
The diagram below shows a flowchart for the investigation of an individual with suspected Antithrombin deficiency.
Click HERE to return to the top of the page.
