Fibrinogen Degradation Products [FDPs]
Introduction
Fibrinogen consists of three pairs of polypeptide chains: two Aα, Bβ and γ. These are linked together by 29 disulphide bonds in such a way that N-terminal regions of the 6-polypeptide chains meet to form a central E-domain. The C-terminal regions [Aα, Bβ and γ] form the D-domain and these are joined by α-helical ropes to the central E-domain to give the characteristic Fibrinogen structure.
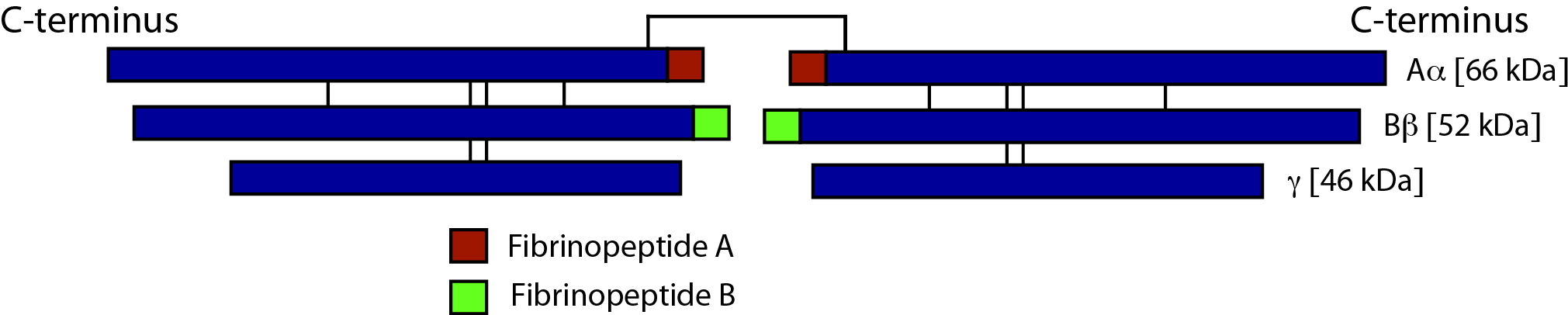
Activation of Fibrinogen by Thrombin [IIa] cleaves the two short peptides from the N-terminal regions of the Aα and Bβ chains - these peptides are known as Fibrinopeptide A [FpA - 16 amino acids] and Fibrinopeptide B [FpB - 14 amino acids] respectively. Removal of the N-terminal sequences from the Aα and Bβ chains chains reveals new N-terminal sequences in the Aα and Bβ chains located within the E domain, known as 'knobs.' These knobs can interact spontaneously with the D-domains to form Fibrin polymers. Under the influence of factor XIIIa, cross-linking of these Fibrin polymers [between Glutamine and Lysine amino acids] occurs to form cross-linked Fibrin polymers.
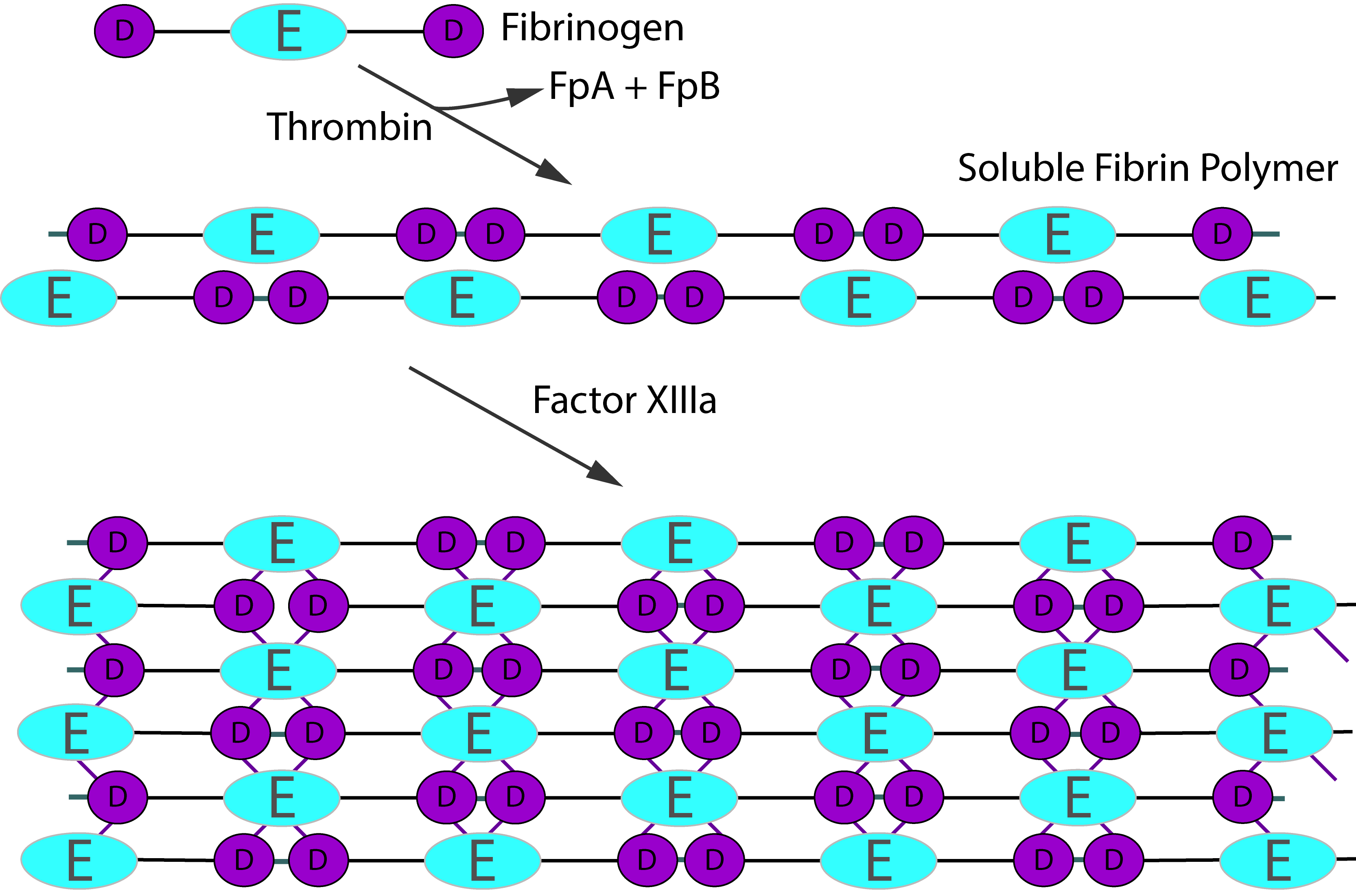
To recap the sequence is:
Fibrinogen → Fibrin monomer → Soluble Fibrin polymer [non cross-linked] → Cross-linked Fibrin polymer
Thrombin activation of Factor XIII is accelerated by the presence of non–cross-linked Fibrin but is inhibited by fully cross-linked Fibrin. This auto-regulation serves to help limit Factor XIIIa activity to sites of Fibrin clot formation. The principal sites of cross-linkage induced by FXIIIa are between the γ-domains in the D domains of adjacent Fibrin monomers and between the carboxyl-terminal ends of the α chains of Fibrin monomers.
Fibrinogen Degradation Products [FDPs]
Fibrinogen Degradation Products [FDPs] consisting of Fragments X, D, Y and E are generated when Fibrinogen or non-cross linked Fibrin [soluble Fibrin] is broken down by Plasmin and are, therefore, fundamentally different from D-Dimers which are generated when cross-linked Fibrin [Fibrin that has been cross-linked by Factor XIIIa] is broken down.
1. Degradation of Fibrinogen and non-cross Linked Fibrin
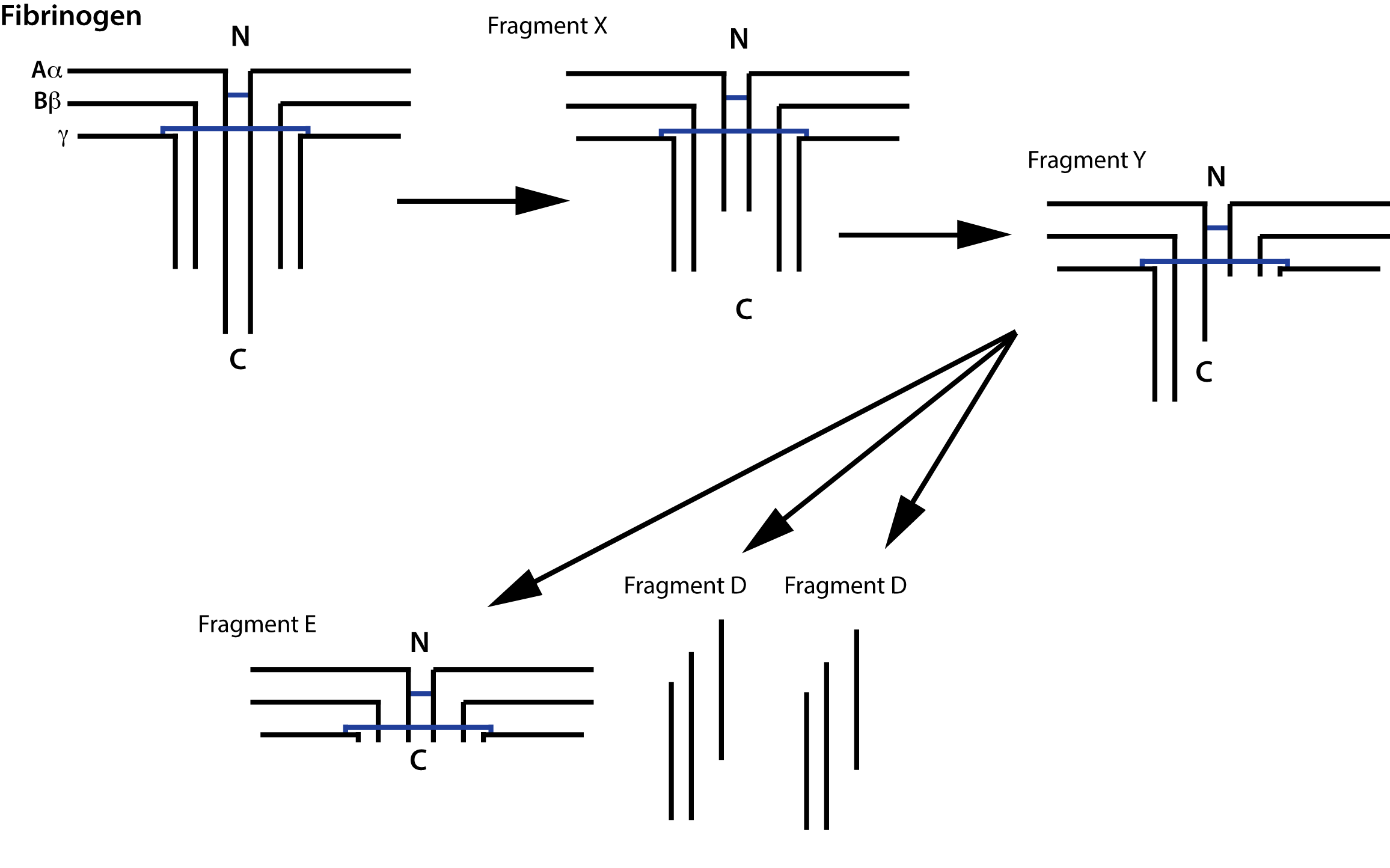
Fibrinogen [and soluble, non-cross linked Fibrin] is degraded by Plasmin generating a series of fragments [see above].
Partial degradation of the γ chain gives rise to Fragment X and then further digestion of this fragment gives rise to Fragment Y. Digestion of Fragment Y gives rise to Fragment E and two D Fragments.
2. Degradation of Cross-Linked Fibrin
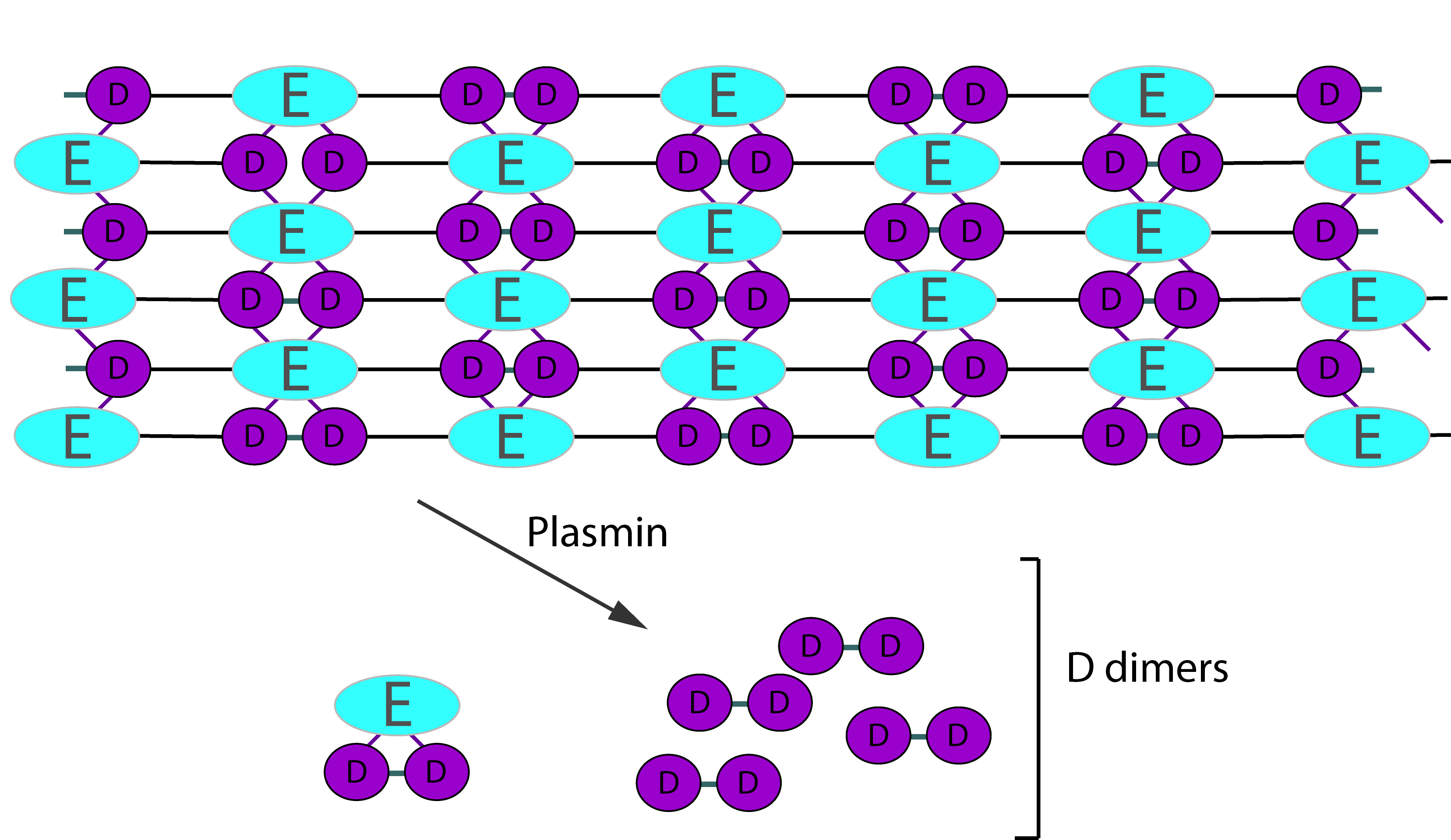
Digestion of cross-linked Fibrin by Plasmin gives rise to a variety of fragments including D-dimers. These define the breakdown of cross-linked Fibrin [i.e. Fibrin that has been cross-linked by FXIIIa] as the cross-linking links D-fragments. Digestion by Plasmin cleaves cross-linked Fibrin in a random order to generate a series of soluble fragments [so called 'X-oligomers'] of varying molecular weights and including D-dimers. In each of these breakdown steps, the γ–γ cross-links are retained and so D-dimers are present. Although the diagram below shows D-dimers in isolation, in practice they exist as a variety of D-Dimer containing oligomers e.g. EDD. The process is dynamic and more fragments are generated as the process proceeds. D-dimers create a neo-epitope and it is this that is detected by specific antibodies used in the various detection systems.
Principles & Methodology of Assays
1. Latex particle/bead agglutination assays
In Latex-based assays, particles are coated with antibodies to purified FDP fragments - usually Fragments D and E. The suspension is mixed with a dilution of the serum sample to be tested and aggregation will occur if FDPs are present. By using differing dilutions of the serum sample, a semi-quantitative assay for FDPs can be performed. Serum FDP assays that use polyclonal antibodies will cross-react with Fibrinogen and this must be removed prior to testing. This can be achieved either by the addition of Thrombin or by the use of specialised collection tubes containing Batroxobin [also known as Reptilase] - a snake venom isolated from the snake Bothrops atrox and inhibitors of Fibrinolysis.
2.
Latex-agglutination assays utilising monoclonal antibodies
In this assay, the use of monoclonal antibodies ensures that antibody does not cross-react with Fibrinogen and so the test can be used on citrated plasma samples.
3. Enzyme immunoassays
A microtitre plate is pre-coated with a Biotin-conjugated antibody specific to FDPs. Standards or plasma samples are added to the appropriate wells and incubated. Avidin conjugated Horseradish Peroxidase [HRP] is added to each well and incubated. An HRP substrate [TMB] is then added and only those wells that contain FDPs, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in colour. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the colour change is measured spectrophotometrically at 450nm. The concentration of FDPs in the samples is then determined by comparing the OD of the samples to a standard curve.
TMB [3,3', 5,5"-Tetramethylbenzidine] is used as a substrate in a number of different assays. The reaction between the TMB substrate and a peroxidase, typically Horseradish Peroxidase [HRP], produces a measurable colour change that correlates with the analyte under investigation - in this case FDPs.
As in all tests, positive and negative controls should be included.
Causes of an Elevation in Fibrinogen Degradation Products [FDPs]
A number of disorders can lead to an elevation in FDPs and these include:
- Disseminated Intravascular Coagulation [DIC]
- Eclampsia
- Malignancy
- Following surgery
- Cardiac disorders
- Hepatic disorders including transplantation
- Thrombolytic therapy e.g. following the use of Streptokinase
- Pulmonary embolism [PE]
- Deep vein thrombosis [DVT]
- Inflammation
