Introduction
Two main alternatives to the 1-stage Factor VIII assay exist, both based around an initial step to generate Factor Xa in a quantity proportional to the Factor VIII concentration and a second step to assay the Factor Xa and from this derive the Factor VIII concentration. In the 2-stage Factor VIII assay the amount of Factor Xa generated is measured by a clotting-based technique whilst in the Chromogenic Factor VIII assay it is measured using a chromogenic substrate.
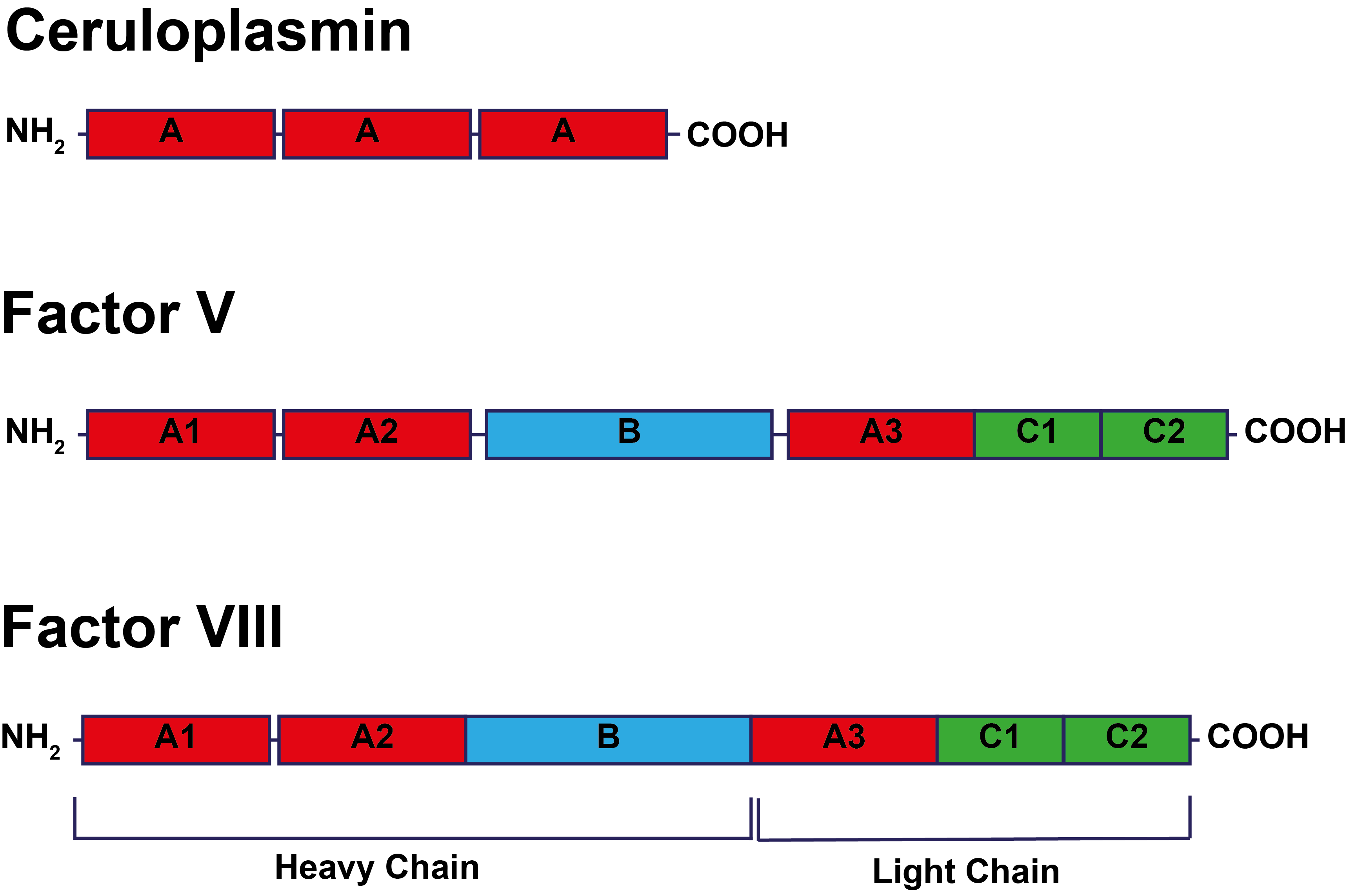
The structure of FVIII and its activation/inactivation are shown below:
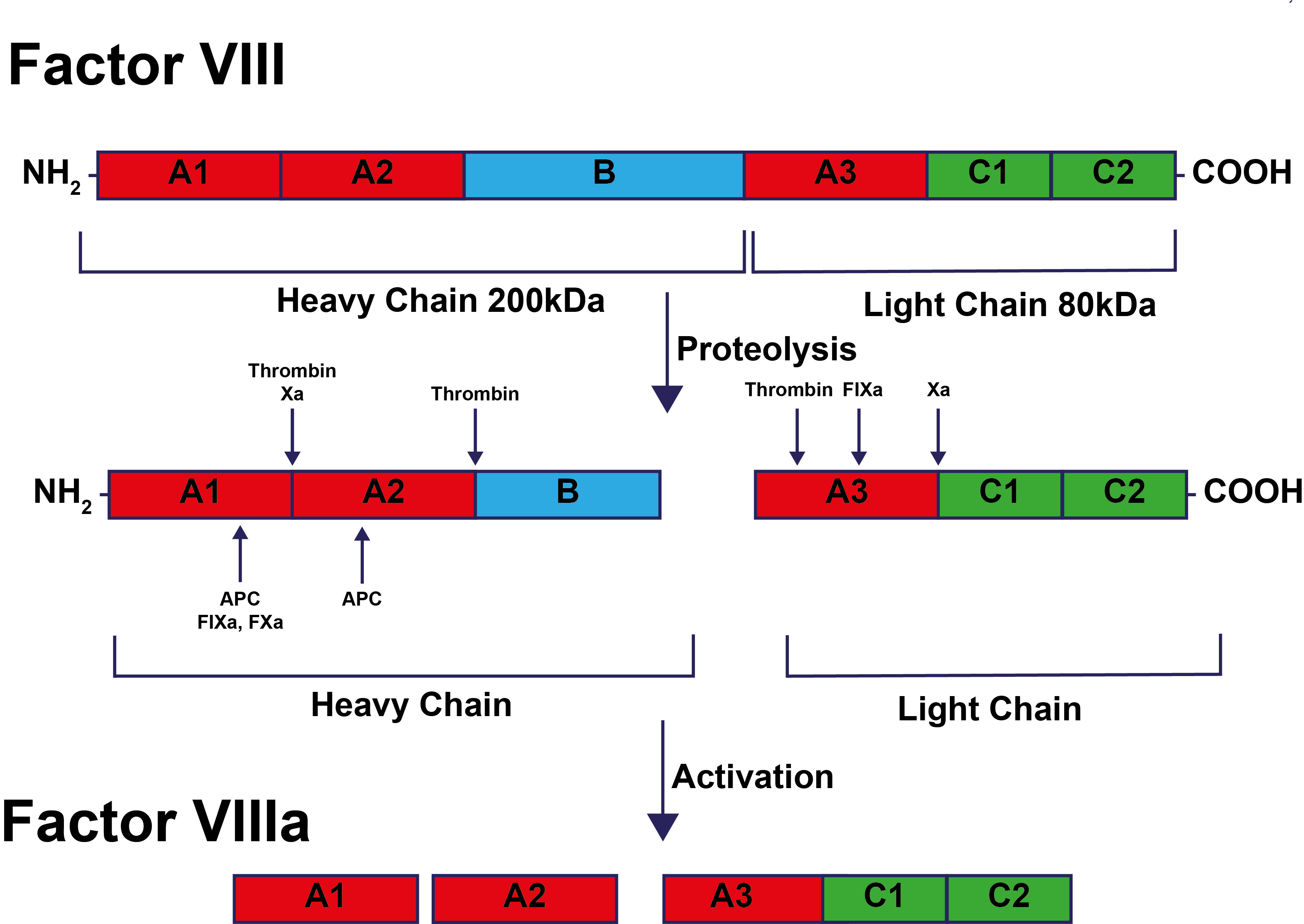
Activation of Factor VIII occurs via cleavage of the heavy
chain at Arg372 and Arg740 and cleavage of the light chain at Arg1689. FVIIIa circulates as a heterotrimer comprising the A1 domain [amino acids 1–372], the A2 domain (amino acids 373–740] and the A3-C1-C2 domains which are linked [amino acids 1690–2332]. The B domain is removed from the active form of FVIIIa. Inactivation of FVIII occurs by cleavage of the A1 and A2 domains at Arg336 and Arg562 by Activated Protein C.
The 2-stage clotting-based Factor VIII assay is less commonly performed than the 1-stage Factor VIII assay and in many labs, the 2-stage FVIII assay has been replaced by the chromogenic FVIII assay.
The 1-stage Factor FVIII assay is simple to perform, easily automated and a relatively inexpensive test. However, the test is susceptible to interference from pre-activation of Factor VIII or the presence of
Antiphospholipid antibodies i.e. a Lupus Anticoagulant - which can give rise to misleading results. In addition there are a number of F8 gene mutations that can lead to discrepant 1-stage/2-stage FVIII assay results. Two types of such discrepancies exits - the classical type in which the 2-stage FVIII assay is lower than the 1-stage assay and the reverse which is less common.
Principles & Methodology
a. 2-Stage Factor VIII Assay
Factor VIII is a cofactor in the coagulation cascade but has no intrinsic enzymatic activity. Therefore, the concentration of Factor VIII is made the rate limiting step in a reaction that generates Factor Xa - the First Stage of the Two Stage Assay. The Second Stage determines the amount of FXa produced.
The 2-stage Factor VIII assay requires:
| Component | Explanation |
|---|---|
| Activated serum | This provides factors IX, X and XIa. XIa initiates coagulation in the patient sample. Commercially available or prepared by incubating whole blood in glass, centrifuging and then removing the serum. |
| Factor V | Commercially available, usually bovine. Factor V is required as a cofactor for the initial coagulation reaction |
| Adsorbed patient plasma | Adsorption with Al(OH)3 removes factors II, VII, IX and X - the vitamin K dependent clotting factors. This prevents progression of the first stage beyond assembly of the Prothrombinase complex |
| Normal plasma | To supply Prothrombin and Fibrinogen so that coagulation can proceed to clot formation |
| CaCl2 and Phospholipid | The reaction is dependent on calcium ions for factor activity and phospholipid surfaces to facilitate factor interactions |
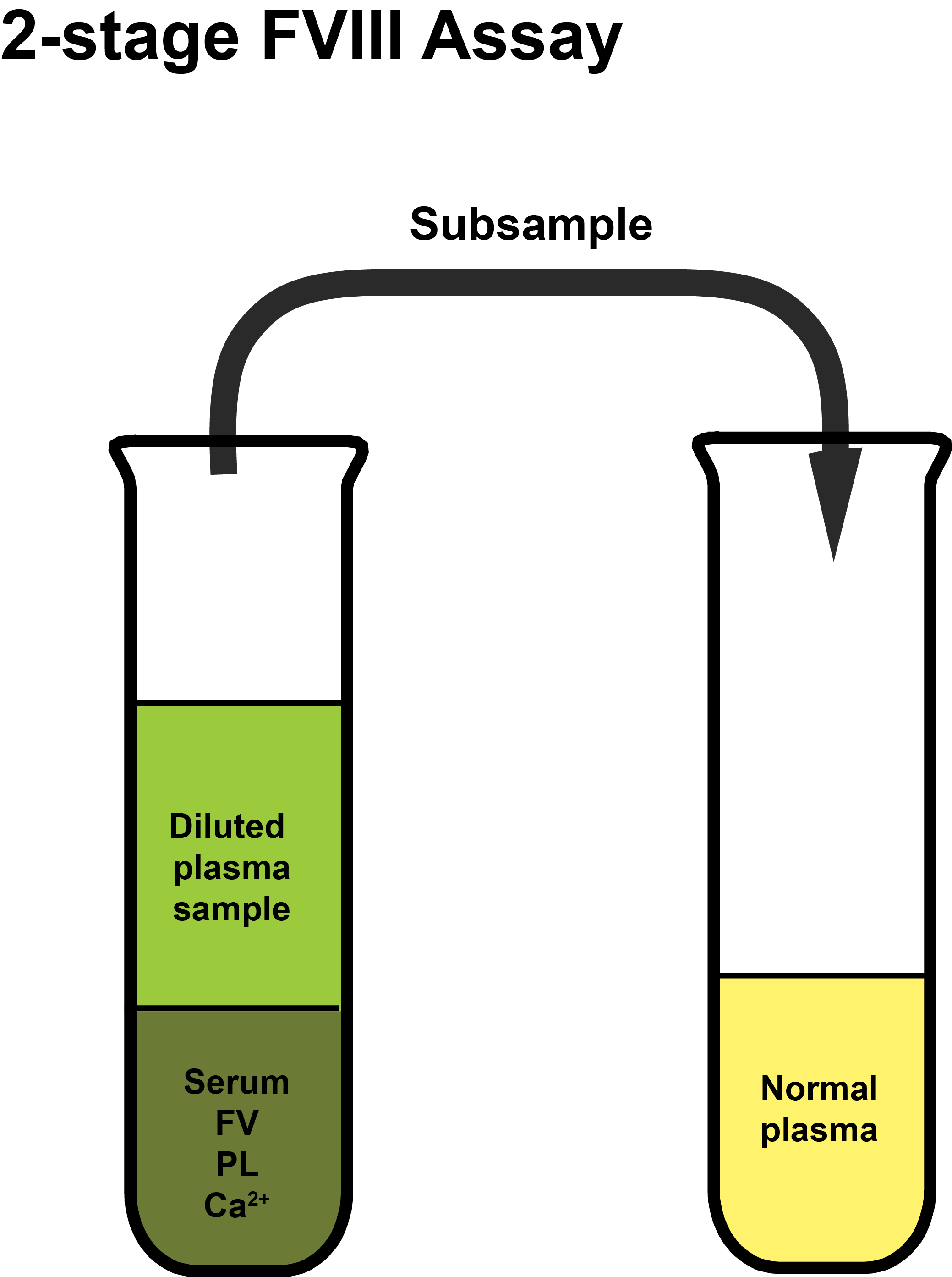
Adsorbed patient's plasma is mixed with activated serum, Factor V, calcium and phospholipid to initiate coagulation and generate Factor Xa. After a fixed period of incubation an aliquot of the mixture is added to an aliquot of normal plasma and the time to clot formation measured. Clotting times are plotted on double log paper and from which, by comparison to a reference curve derived using a reference plasma sample with a known Factor VIII concentration, the Factor VIII level in the patient sample can be derived.
The patient’s plasma is adsorbed to remove Prothrombin [Factor II] and so prevents clot formation when the coagulation cascade is initiated.
Stage 1: Coagulation is initiated by the addition of Factor XIa whilst factor X and V are provided in excess. The Factor VIII concentration is, therefore, the rate limiting step in the formation of Factor Xa.
Stage 2: In the second stage, a sample of the first stage mixture is added to normal plasma and the time to clot formation recorded. Since the clotting time will be dependent on the Factor Xa level in the sample from the first stage and the Factor Xa level is proportional to the concentration of Factor VIII in the patient’s plasma, the Factor VIII level may, therefore, be derived from the clotting time of the second stage.
In the schematics shown below, the principles of the 2-stage FVIII assay are illustrated. The plasma sample used to generate Factor Xa and which is then sub-sampled into normal plasma, cannot clot as the Prothrombin has been removed by the Al(OH)3 adsorption step.
b. Chromogenic Factor VIII Assay
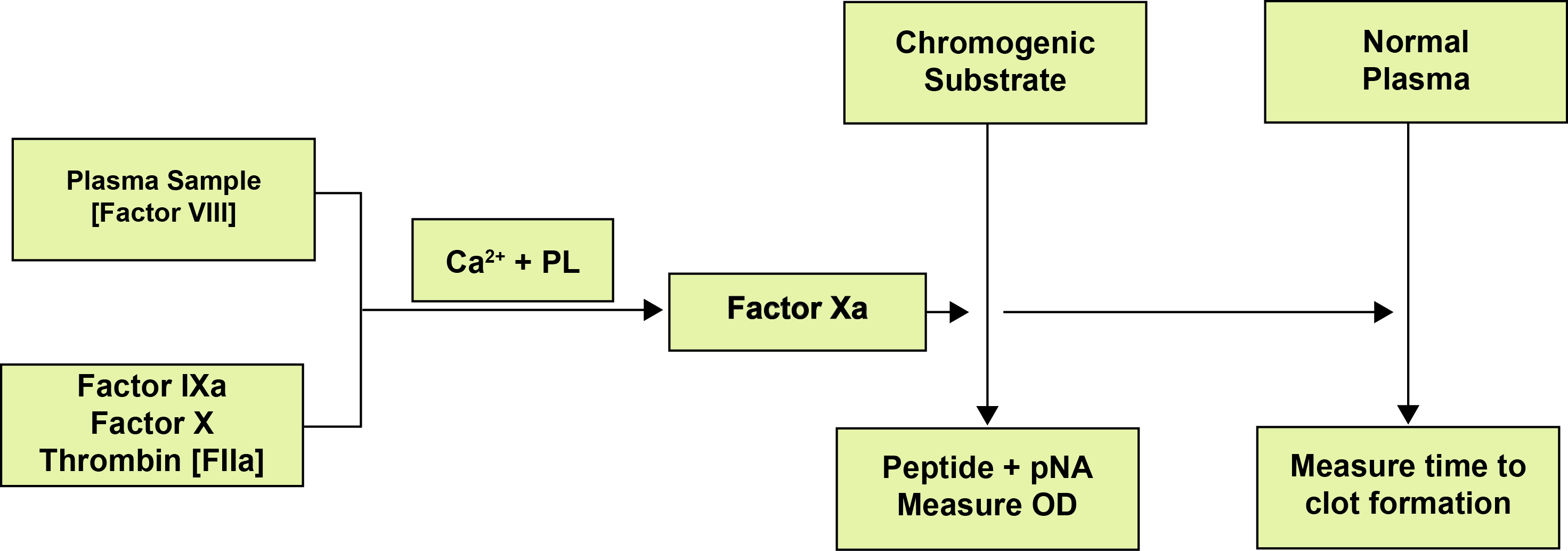
The assay is similar to the Two-stage Factor VIII assay in that it involves an incubation step to generate Factor Xa and a second stage to determine the amount of Factor Xa produced. In this case the amount of Factor Xa is measured by its action on a specific chromogenic substrate and since the colour intensity produced is directly proportional to the amount of FXa, which in turn is directly proportional to the amount of FVIII, the FVIII levels may be calculated from the absorbance of the sample at a specific wavelength (the optimal absorbance wavelength for the chromophore produced by FXa cleavage of the chromogen usually 405nm.
A chromogenic Factor VIII assay requires:
| Component | Explanation |
|---|---|
| Reagent cocktail for generating FXa | Contains FIXa, FX in excess, Thrombin [Factor IIa], a source of calcium ions and phospholipid |
| Chromogenic substrate | A substance cleaved by FXa to produce a colour change. May also contain a Thrombin inhibitor to stop the FXa generation when the chromogen is added |
| Patient plasma | Platelet poor plasma |
The patient’s plasma is incubated with the reagent cocktail at 37°C. The Thrombin in the cocktail activates the FVIII to FVIIIa and, in the presence of Ca2+ and phospholipid, this acts as a co-factor for FIXa for the conversion of FX to FXa. The FVIII concentration is the rate limiting step. The chromogenic substrate is added and Factor Xa hydrolyses the chromogenic substrate and the absorbance of the resulting product (generally p-nitroaniline released enzymatically) is measured and compared to a reference curve to give the FVIII level.
A reference curve is drawn by plotting the absorbance [usually at 405nm] against concentration on linear graph paper as shown below. For example - if in the case of an unknown plasma sample the absorbance at 405nm was 0.2 - then from the graph below the concentration of FVIII:C is 56 IU/dl.

The data used to construct the graph is shown in the table below:
| Concentration [IU/dL] | Absorbance [405nm] |
|---|---|
| 140 | 0.44 |
| 100 | 0.32 |
| 50 | 0.19 |
| 0 | 0.04 |
Interpretation
See Comments for the 1-stage/2-stage FVIII discrepancies.
Reference Ranges
Reference ranges are expressed in IU/dL or IU/mL.
The Factor VIII reference range is usually 0.50-1.50 IU/ml or 50-150 IU/dL. The reference range for neonates is very similar to that of adults.
Factors V, VIII and Ceruloplasmin.
Factors V, VIII and Ceruloplasmin [the major copper carrying protein in the blood] share similarities in structure suggesting they may have originated from a common ancestral protein.
Click HERE to return to to the top of the page.
